Abstract
Introduction. Persistent thrombocytopenia (PT) due to delayed primary platelet recovery or secondary graft failure with prolonged thrombocytopenia is a relevant and relatively common complication after allogeneic stem cell transplantation (alloSCT). It can result in transfusion dependence and hemorrhagic events, leading to increase of morbidity and mortality. Concomitant causes of PT after alloSCT are several and can include viral infections, graft-versus-host disease (GvHD), drug toxicity, thrombotic microangiopathy (TMA) and immune thrombocytopenia (ITP). Together with the treatment of the primary cause and the support with platelet transfusion, the use of thrombopoietin receptor agonists (TPO-RAs), which proved to be effective in several scenarios (e.g. ITP or aplastic anemia), is gaining a fundamental role also in the post alloSCT setting. To date, the use of TPO-RAs after transplant has been investigated in small studies or case reports. In this study, we evaluated the use of romiplostim in a larger patient cohort to determine its safety, efficacy and potential predictors of response.
Materials and methods. We conducted a retrospective study on consecutive patients undergoing alloSCT and receiving romiplostim for the treatment of PT at the bone marrow transplantation unit of the University Hospital of Ulm. PT was defined as a platelet count < 20 Giga/l for 7 consecutive days after engraftment, the need for continuous transfusion during the post-transplant follow-up or a not rapidly reversible decrease of >50% of the platelet count not due to relapse of underlying hematological disease. Response to treatment was defined as a platelet count >50 Giga/l for at least 7 consecutive days without transfusion. Patients with residual bone marrow involvement of primary hematological disease were excluded. Univariate analysis was conducted using Fisher's exact test or Mann-Whitney-U-test.
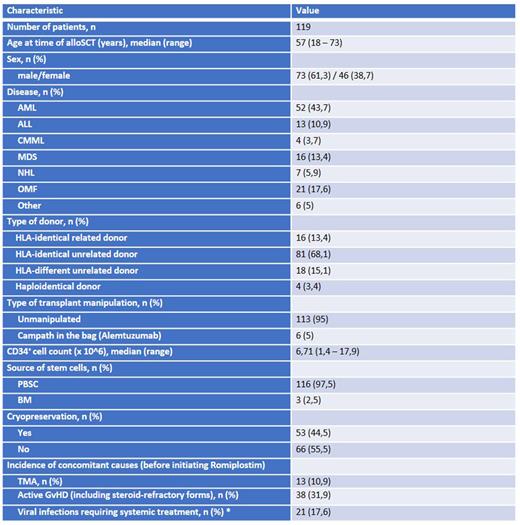
Results. Between 01/2019 and 12/2021 we identified a total of 119 out of 255 transplanted patients (46,7%) with PT receiving, as a part of the treatment, romiplostim during the post-transplant follow-up. Patient characteristics are listed in Table 1. The median platelet count at baseline was 32 Giga/l (r = 0 - 88). We started with romiplostim 250μg on a weekly basis, in 46,2% of cases the dose was escalated to 500 µg during treatment. Seventy-three percent showed a clinical response to treatment with a median time to response of 40 days (r = 7 - 565). We observed adverse events like thrombosis, pain syndrome, dizziness in only 8,4% of patients. The median duration of treatment was 168 days (r = 18 - 761). In patients with response, the median platelet count was 91 Giga/l (r = 53 - 212). In univariate analysis, we evaluated potential risk factors associated with poor response to romiplostim. While the presence of a viral reactivation (like CMV, EBV, BKV, HSV1) requiring systemic treatment was associated with a significant lower response rate (47.6% versus 79.6%, respectively for patients with or without reactivation, p= 0.005), the presence of active GvHD (also in case of steroid-refractory (SR) forms), of TMA and the number of CD34-positive stem cells of the graft did not play a role in determining the efficacy of romiplostim. We also evaluated the influence of graft cryopreservation, which was performed increasingly during the SARS-CoV-2 pandemic, on the rate of efficacy of romiplostim in PT treatment. We could not identify a significant difference as compared to the non-cryopreserved grafts.
Conclusion. Our results indicate that romiplostim is overall well tolerated and represents a reasonable and effective treatment for PT after alloSCT. We observed that viral infections requiring systemic antiviral treatment could represent a predictor of worse response, which could potentially be explained by the myelotoxicity induced not only by the virus itself but also as a consequence of antiviral substances. Of note, especially considering that SR GvHD or TMA did not seem to impair the efficacy of romiplostim, the stimulation of TPO receptor could play a role in reducing the rate of morbidity and mortality associated with such severe complications of the post transplant follow-up. Also, cryopreservation did not impact on the rate of success of TPO-RAs based treatment strategy. These preliminary results need to be further validated in larger and prospective cohorts.
Disclosures
Döhner:Janssen Pharmaceuticals: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Syndax Pharmaceuticals Inc.: Consultancy, Honoraria; Gilead Sciences, Inc.: Consultancy, Honoraria; Agios Pharmaceuticals: Consultancy, Honoraria, Research Funding; Pfizer Inc.: Research Funding; Novartis AG: Consultancy, Honoraria, Research Funding; AbbVie Inc.: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Brystol Myers Squibb: Consultancy, Honoraria, Research Funding; Daiichi Sankyo Co, LTD: Consultancy, Honoraria; AstraZeneca: Honoraria; Kronos Bio, Inc.: Research Funding; Amgen Inc.: Consultancy, Honoraria, Research Funding; Berlin-Chemie AG: Consultancy, Honoraria; Astellas Pharma Inc.: Consultancy, Honoraria, Research Funding. Sala:Takeda: Consultancy; Novartis: Honoraria; Kite Gilead: Consultancy, Honoraria, Other: Support for meeting attendance; Jazz Pharmaceutical: Consultancy, Honoraria, Other: Support for meeting attendance; BMS: Consultancy, Honoraria.
OffLabel Disclosure:
Romiplostim for persistent thrombocytopenia after allogeneic stem cell transplantation
Author notes
Asterisk with author names denotes non-ASH members.